Suboxone Lawsuit Explodes, 1,882 Victims Already Filed Lawsuit, See If You Qualify & How Much Your Case Could Be Worth
The Suboxone lawsuit alleges manufacturers Indivior and Aquestive failed to warn users that their opioid addiction treatment causes severe dental damage, including tooth decay, tooth loss, cavities, and gum disease. Over 1,882 claims are now pending in federal court, with victims seeking compensation for dental injuries that manufacturers knew about but concealed for over a decade. A critical June 2025 statute of limitations deadline looms for many states with three-year filing windows, forcing victims to act immediately or lose their right to sue.
What Is the Suboxone Lawsuit About?
Suboxone, a sublingual film containing buprenorphine used to treat opioid use disorder, causes catastrophic dental problems because of its highly acidic formulation with a pH of 3.4. Lawsuits claim Indivior failed to warn about severe dental issues related to design defects until the FDA mandated label changes in June 2022—over a decade after the product launched.
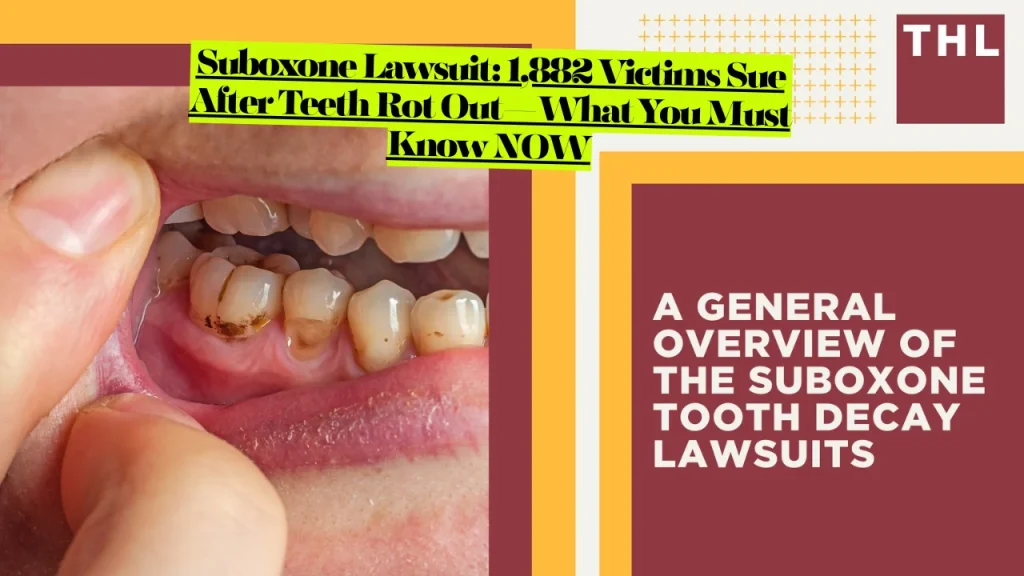
The FDA issued its first warning in January 2022 after identifying 305 cases of dental problems, including tooth decay, cavities, dental abscesses, tooth erosion, and total tooth loss, even in patients with no prior dental history. Of these cases, 71 required tooth extraction or removal, with some reports documenting involvement of all teeth or “rampant decay” affecting 11 or more teeth.
Related lawsuit: Walmart Class Action Lawsuit Recent November 45 Million Settlement Payments Updates, Radioactive Shrimp Claims, and Employment Violations

Who Can File a Suboxone Lawsuit?
You may qualify if you:
- Used Suboxone sublingual film or generic buprenorphine/naloxone strips
- Experienced severe dental problems including tooth decay, tooth loss, cavities, gum disease, or dental erosion
- Required extensive dental work such as extractions, root canals, crowns, or implants
- Used the medication before June 2022 when warnings were finally added
- Can document your Suboxone use and dental damage through medical records
Plaintiffs must submit detailed information through the Rubris Crosslink system to verify brand-name Suboxone Film usage and dental injuries linked to the medication.
What Dental Problems Did Suboxone Cause?
The FDA review revealed dental problems occurred as soon as two weeks after beginning treatment, with median diagnosis time around two years after starting therapy. Reported injuries include:
- Severe tooth decay and cavities affecting multiple teeth simultaneously
- Complete tooth loss requiring full extractions and dentures
- Dental erosion destroying protective enamel from acid exposure
- Gum disease and oral infections from bacterial changes
- Tooth fractures making teeth structurally unsound
- Root canals, crowns, and implants costing tens of thousands
User testimonials reveal devastating consequences: “I’m 45 years old and I’ve got 24 teeth that have broke off even with the gums. I’ve been on Suboxone for over 12 years”.
Why Didn’t Manufacturers Warn About Dental Risks?
Plaintiffs allege manufacturers knew about the acidic formulation’s dental risks but chose profits over patient safety, failing to provide adequate warnings despite mounting evidence. The court allowed failure-to-warn claims to proceed, emphasizing that Suboxone’s label failed to address dental injury risks until 2022, leaving patients and doctors uninformed about potential harm.
Suboxone had been on the market since 2002 without warnings, potentially exposing many patients to preventable tooth damage for two decades. The core allegation is that defendants knew of severe tooth decay risks but didn’t convey that risk to prescribing doctors or patients.
What Is the Current Status of the Suboxone MDL?
All federal Suboxone lawsuits are consolidated in MDL No. 3092 in the Northern District of Ohio before Judge J. Philip Calabrese, established in February 2024. Key developments:
January 2025: Judge Calabrese partially denied Indivior’s motion to dismiss, allowing lawsuits related to design defects and failure to warn about severe dental issues to move forward.
September 2025: Case numbers surged from 889 to 1,882 claims in one month—more than double—as awareness spread and filing deadlines approached.
October 2025: A court order requires Indivior and Aquestive to produce extensive internal records, including FDA filings, marketing materials, and adverse-event reports on nearly 90 dental injuries.
Bellwether trials are expected to begin in late 2025 or early 2026, which will guide future settlement negotiations and help both parties gauge claim strength.
What Evidence Do You Need to File a Claim?
Building a strong Suboxone case requires comprehensive documentation:
Medical Records:
- Dental records showing pre-Suboxone oral health status
- Treatment records documenting dental damage timeline
- Diagnostic reports linking damage to Suboxone use
Prescription Documentation:
- Pharmacy records proving Suboxone sublingual film use
- Prescription dates establishing duration of exposure
- Brand verification (Suboxone Film vs. generic products)
Financial Documentation:
- Dental treatment invoices for extractions, crowns, implants
- Insurance claims and out-of-pocket expenses
- Projected future dental care costs from dentist estimates
Census protocols require plaintiffs to submit key information, authorizations, and records to establish verified Suboxone Film usage and dental injuries.
What Compensation Can Victims Receive?
Average Suboxone lawsuit settlement amounts are projected to range from $10,000 to $500,000, with minor dental damage cases settling for $10,000-$50,000, moderate cases for $50,000-$150,000, and severe cases requiring extensive dental work potentially reaching $150,000-$500,000 or more.
Settlement compensation typically covers:
- Economic damages: Past and future dental treatment costs, medical expenses, lost wages
- Non-economic damages: Pain and suffering, emotional distress, loss of quality of life
- Punitive damages potential: The potential for punitive damages looms large in this litigation, with trial values potentially exceeding $1 million for strong cases with sympathetic plaintiffs
Settlement projections are based on prior mass tort lawsuits involving dangerous drugs and represent estimates, not guarantees, as no settlements have been reached yet.
What Is the Statute of Limitations Deadline?
The Suboxone MDL faces a critical June 2025 deadline for individuals in states with three-year statute of limitations periods—potentially their last chance to file claims. The Discovery Rule can extend limitations from when a person discovers their injury and knows it was caused by negligence, but failure to file within the statute of limitations can bar you from filing entirely.
With no agreement on tolling measures between plaintiffs and defendants, each claimant must file separately before the deadline. Attorneys may assist in entering tolling agreements to suspend the statute of limitations and protect your ability to file, accommodating delays in securing medical and dental records.
How Do You Join the Suboxone Lawsuit?
Step 1: Gather Documentation Collect dental records, Suboxone prescription history, and treatment expenses before consulting an attorney.
Step 2: Consult a Qualified Attorney Contact a Suboxone lawyer for a free consultation to evaluate your claim strength and filing timeline. Attorneys work on contingency, meaning no fees unless they secure compensation.
Step 3: File Your Claim Cases can be filed directly in the MDL court through a streamlined Short Form Complaint process, bypassing transfers from different U.S. District Courts.
Step 4: Complete Census Protocols Submit required information through the Rubris Crosslink system to verify your Suboxone use and dental injuries.
Recent Court Rulings Affecting Suboxone Claims
On December 31, 2024, the U.S. District Judge overseeing federal Suboxone lawsuits denied the drug manufacturer’s motion to dismiss certain failure-to-warn claims, rejecting arguments that federal regulations restricted their ability to independently update label warnings.
The court ruled that plaintiffs can pursue failure-to-warn claims for actions after June 2022, alleging manufacturers could have strengthened warnings earlier under FDA regulations as new evidence about dental risks emerged. A December 2022 JAMA research letter found users of sublingual buprenorphine had more than twice the rate of dental problems compared to other forms of the drug.
Why This Lawsuit Matters
Unlike some side effects, dental risks can often be mitigated through preventive measures like rinsing the mouth after use, increasing fluoride intake, or scheduling more frequent dental checkups. By failing to provide adequate warnings, Suboxone manufacturers deprived patients of the ability to take proactive steps to protect their health.
The FDA recommends patients take a large sip of water after Suboxone film dissolves, swish it gently around teeth and gums, and wait at least one hour before brushing to avoid enamel damage. Individuals should also schedule dental appointments immediately after prescription, inform dentists of Suboxone use, request tooth decay prevention plans, and maintain regular checkups.
Had manufacturers disclosed these risks earlier, thousands of patients could have prevented catastrophic dental damage through simple protective measures.
Frequently Asked Questions
Q: What is the Suboxone lawsuit about?
The lawsuit alleges manufacturers knew Suboxone’s acidic formulation caused severe tooth decay and dental injuries but failed to warn prescribing doctors or patients because they chose profits over people.
Q: What dental problems qualify for a Suboxone claim?
Qualifying dental problems include tooth decay, cavities, dental abscesses/infection, tooth erosion, and total tooth loss—even in patients with no history of dental issues before using Suboxone.
Q: Who can file a Suboxone lawsuit?
Anyone who used Suboxone sublingual film or generic buprenorphine/naloxone strips and experienced severe dental damage can file. Allegations center on the sublingual film version, not injectable or patch forms.
Q: How much compensation can I get from a Suboxone lawsuit?
Settlement projections range from $10,000 to $500,000 depending on damage severity, with trial verdicts potentially reaching $500,000 to $1 million in strong cases.
Q: What evidence do I need for a Suboxone lawsuit?
You need dental treatment invoices, medical records showing before/after dental health, pharmacy records proving Suboxone use, and projected future dental care estimates from your dentist.
Q: What is the deadline to file a Suboxone claim?
Many states face a June 2025 deadline for three-year statute of limitations periods—potential claimants must act quickly to meet filing deadlines and secure compensation.
Q: How do I join the Suboxone lawsuit?
Contact a qualified Suboxone attorney for a free consultation—lawyers are still accepting new claims as of November 2025, though some firms are cutting off new cases as deadlines approach.
Legal Disclaimer: This information is for educational purposes only and does not constitute legal advice. If you believe you have been harmed by Suboxone, consult with a qualified attorney to discuss your specific situation and legal options.
Sources:
- FDA Safety Warning on Buprenorphine Dental Risks (January 2022)
- MDL No. 3092 Case Documents, Northern District of Ohio
- Recent court rulings on Indivior motion to dismiss (January 2025)
Related Articles:
- Depo-Provera Brain Tumor Lawsuit: File NOW Before Statute of Limitations Expires
- DePuy Attune Knee Lawsuit: 400,000 Implants at Risk—Active Claims, Zero MDL
- Facebook Lawsuit Payout: Active Settlements, Eligibility & Payout Amounts
About the Author

Sarah Klein, JD, is a licensed attorney and legal content strategist with over 12 years of experience across civil, criminal, family, and regulatory law. At All About Lawyer, she covers a wide range of legal topics — from high-profile lawsuits and courtroom stories to state traffic laws and everyday legal questions — all with a focus on accuracy, clarity, and public understanding.
Her writing blends real legal insight with plain-English explanations, helping readers stay informed and legally aware.
Read more about Sarah
