Depo-Provera Brain Tumor Lawsuits Explode, 1,346 Women File Claims as FDA Rejection Fuels $500K+ Settlement Fight
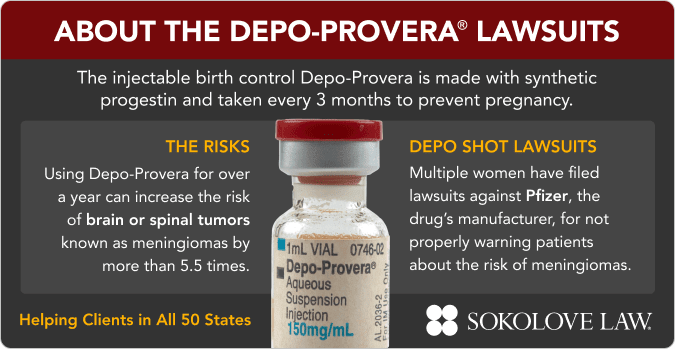
Over 1,346 Depo-Provera lawsuits have been filed in federal court as of October 2025 by women who developed meningioma brain tumors after using the birth control shot, with an additional 439 cases pending in state courts including Delaware (332), New York (78), and California (20). Women who used Depo-Provera for over one year face 5.5 times higher risk of developing meningiomas according to scientific studies, yet Pfizer still hasn’t added brain tumor warnings to U.S. labels despite updating warnings in Europe and Canada.
Lawyers estimate settlement compensation between $150,000 and $800,000 depending on injury severity, with cases surging 122% over two months—jumping 46% from August to September, then 52% from September to October 2025.
What Is the Depo-Provera Lawsuit About?
The multidistrict litigation (MDL No. 3140) centralizes claims against Pfizer in the Northern District of Florida under Judge M. Casey Rodgers, with women alleging Pfizer knew about meningioma risks but failed to warn patients. A September 2025 JAMA Neurology study found women using Depo-Provera were 2.45 times more likely to develop intracranial meningiomas than women on other birth control.
The litigation centers on Pfizer’s alleged decades-long knowledge of brain tumor risks. Studies linking Depo-Provera to meningioma date back to 1983 when researchers discovered synthetic hormones could stimulate progesterone receptors in these tumors. European regulators required Pfizer to add meningioma warnings, and Canada followed suit, yet U.S. labels remain unchanged.
Who Can File a Depo-Provera Claim?
You may qualify if you:
- Used Depo-Provera, Depo-SubQ Provera 104, or authorized generics for at least one year (4+ injections)
- Developed an intracranial or spinal meningioma after starting injections
- Underwent surgery, radiation, or have treatment scheduled
- Were diagnosed with meningioma-related symptoms including seizures, vision loss, headaches, or neurological damage
As of November 2025, 1,225 cases are pending in the federal MDL 3140 before Judge M. Casey Rodgers. Plaintiffs’ lawyers report holding nearly 10,000 additional unfiled claims, suggesting the litigation could expand significantly.
Related Lawsuit: Tisha Campbell and Martin Lawrence Lawsuit, Wedding Photos Go Viral, Why Fans Are Digging Up the 1997 Scandal That Ended Martin
Critical September 2025 Preemption Battle
Pfizer filed a motion for summary judgment claiming FDA’s 2024 rejection of its proposed meningioma warning prevents state failure-to-warn lawsuits under federal preemption doctrine. Oral arguments were heard September 29, 2025, before Judge Rodgers, who will decide whether thousands of cases can proceed or face dismissal.
Plaintiffs countered September 22, 2025, arguing Pfizer’s proposed label was so broad the FDA had no choice but to reject it. They claim Pfizer deliberately submitted vague language lacking scientific specificity, then used the rejection as a legal shield. Plaintiffs assert Pfizer misrepresented or withheld evidence from the FDA and could have strengthened warnings independently.
This preemption ruling determines whether the MDL survives. If Pfizer prevails, most cases could be dismissed before discovery. If plaintiffs win, litigation advances to bellwether trials potentially starting late 2026 or early 2027.
Proof of Use Requirements Now Streamlined
Judge Rodgers issued Pretrial Order No. 22 on May 6, 2025, requiring all plaintiffs to complete a “Plaintiff Proof of Use/Injury Questionnaire” within 120 days of March 14, 2025, or within 120 days of filing for new cases.
The order compels third parties—pharmacies, clinics, hospitals, insurance companies, and military medical providers—to provide records confirming Depo-Provera injections, even when traditional medical records are unavailable. Many women received injections over a decade ago when records were lost, destroyed, or didn’t identify the specific DMPA manufacturer.
September 2025 court changes allow women to remain in the litigation even without concrete records initially, provided they meet specific requirements. This expansion significantly increases potential claimant numbers.
Five Bellwether Cases Already Selected
Five Depo-Provera cases have been selected to serve as bellwether trials, designed to test claim strength before juries. These include:
Blonski v. Pfizer Inc.: Allison Blonski began using Depo-Provera in 2002. After experiencing severe headaches and arm twitching, doctors discovered a meningioma. Nearly a decade later, a second meningioma was diagnosed.
Sharon Infanti used Depo-Provera for a decade, stopping in 2005. She developed worsening speech difficulties and eventually underwent surgery and radiation for meningioma.
Jennifer Childers received her first Depo shot at age 16 in 1998, continuing until 2007. After suffering vertigo, she was diagnosed with meningioma in 2018 but only recently learned of the drug link.
Judge Rodgers directly selected pilot cases rather than allowing lengthy bellwether selection sparring between parties, potentially accelerating the MDL timeline significantly. Trial dates suggest first bellwether cases may go before juries in late 2026 or early 2027.
What Evidence Supports Brain Tumor Claims?
A BMJ study found women using Depo-Provera for over a year were 5.6 times more likely to develop meningioma requiring surgery. When applying this 5.5x increased risk, estimated annual meningioma cases among Depo-Provera users jump to 1,045-1,568 cases per year.
A September 2025 JAMA Neurology study analyzing over 10 million women found those using injectable birth controls with depot medroxyprogesterone acetate had statistically significant increased relative risk of meningioma diagnosis.
The litigation gained traction after a July 2025 Nebraska lawsuit alleged a woman developed two meningiomas requiring invasive brain surgeries in 2023 after prolonged Depo-Provera use. Sandra Somarakis had one meningioma removed in 2009 but continued injections because no one warned her about the Depo connection. She developed another tumor the following year requiring more invasive treatment.
Projected Settlement Amounts and Compensation
No global settlement has been announced, but legal experts project compensation based on similar pharmaceutical litigation:
High-Tier Cases (Surgery Required): Women requiring brain surgery for meningioma removal fall into the highest payout category, with estimated settlement values between $275,000 and $800,000.
Mid-Tier Cases (Radiation/Treatment): King Law estimates potential settlements ranging between $150,000 and $500,000 based on injury severity and provable damages.
Lower-Tier Cases (Monitoring Required): Women with smaller or asymptomatic tumors requiring ongoing monitoring may qualify for tens of thousands in compensation.
Past meningioma settlements have averaged over $800,000, with jury verdicts exceeding $3 million according to the National Library of Medicine. The average meningioma trial verdict is $3.4 million according to research published in Neurosurgical Focus.
Pfizer paid over $2 million in a 2021 Canadian class action settlement for Depo-Provera bone density loss, establishing precedent for Depo-related compensation.
What Damages Influence Settlement Value?
Key factors determining individual compensation:
- Duration of Use: Long-term Depo-Provera use (multiple years, 8+ injections) increases risk and potential awards
- Tumor Severity: Inoperable tumors, multiple meningiomas, or tumors requiring repeat surgeries command higher settlements
- Permanent Injuries: Meningioma symptoms significantly disrupt lives, leading to chronic pain, vision impairment, fatigue, cognitive difficulties, seizure disorders, and neurological damage
- Medical Expenses: Surgery costs, radiation therapy, ongoing monitoring, and future care needs
- Lost Wages: Inability to work, reduced earning capacity, and career disruption
- Quality of Life: Impact on daily activities, relationships, and independence
Brain tumor treatment expenses may exceed $700,000 before insurance, making medical cost recovery critical for many plaintiffs.
Current MDL Status and Timeline
At an October 27, 2025 case management conference, Judge Rodgers met with judges from New York and Delaware state courts to coordinate case matters and move litigation forward efficiently.
Preemption motions scheduled for late 2025 may determine whether many cases are dismissed. Discovery related to preemption closed July 25, 2025, with summary judgment motions due August 22 and oral arguments September 29, 2025.
Discovery on general causation continues into late 2025, with bellwether trials anticipated in 2026. The litigation’s success hinges heavily on medical causation, with Daubert hearings in spring 2025 excluding only the most unsupported expert opinions.
State Court Cases Add Pressure
A New Jersey woman filed directly in the MDL alleging she developed intracranial meningioma after more than a decade of Depo-Provera use, diagnosed in 2023 with lasting harm after brain surgery.
A Delaware complaint includes 100 plaintiffs who opted to file outside federal MDL, with Delaware’s status as Pfizer’s state of incorporation potentially offering strategic advantages for faster proceedings. State court activity in New York (61 cases) and California (11) raises the possibility that one state case could proceed to trial before federal bellwether trials, potentially setting early settlement expectations.
Pfizer’s Defense Strategy
Pfizer argues the FDA rejected its proposed meningioma warning label in 2024, claiming federal law prevents state failure-to-warn lawsuits. A Pfizer spokesperson stated the FDA’s decision “precluded Pfizer from changing the Depo-Provera label and should preempt plaintiffs’ attempt to end-run FDA’s determination”.
Plaintiffs claim Pfizer drafted a label update so broad it applied to numerous products, ensuring FDA rejection. The lawsuit scrutinizes Pfizer’s marketing of the 150mg/mL formulation over the safer, lower-dose 104mg subcutaneous variant, alleging no meaningful effort to promote the safer alternative.
Preemption defenses are common pharmaceutical tactics but rarely successful. Many legal experts believe Pfizer will ultimately be held liable given the international warning precedents and scientific evidence strength.
What Documents Do You Need to File?
Essential documentation includes:
- Complete medical records showing Depo-Provera injections with dates
- Pharmacy records confirming prescription fills
- Insurance claims and explanation of benefits showing Depo administration
- Meningioma diagnosis records including imaging (MRI/CT scans)
- Surgical reports and pathology results if surgery occurred
- Radiation or treatment records
- Ongoing monitoring documentation
- Witness statements from healthcare providers
- Employment records showing lost wages
- Medical bills and expense documentation
Plaintiffs must answer questionnaires under penalty of perjury, with responses treated as formal interrogatory responses under Federal Rules of Civil Procedure.
How Long Until Settlements?
In many mass tort MDLs, settlements are reached before bellwether trials as defendants understand trial verdicts carry massive jury award risk and potential to shift negotiation dynamics favoring plaintiffs.
Few claimants in this MDL will go to court; most will receive tiered settlement offers based on injury severity once preemption battles conclude and bellwether trial results emerge.
First bellwether verdicts are expected mid-to-late 2026, with aggregate settlements likely after initial trial rounds. Early settlement for “clear” injury cases remains possible before trials conclude.
The timeline depends heavily on the preemption ruling. If Judge Rodgers allows cases to proceed, settlement negotiations intensify as trial dates approach. If preemption succeeds, litigation could face significant delays or dismissal.
Similar Pharmaceutical Cases Provide Context
The Depo-Provera litigation follows patterns from other major pharmaceutical MDLs where scientific evidence of harm ultimately led to substantial settlements despite initial manufacturer defenses.
Historically in mass torts, 5-20% of potentially injured claimants file lawsuits. With estimated annual meningioma cases between 1,045-1,568, potential claim numbers could reach 5,000-10,000 if filing rates follow historical patterns.
The MDL structure allows efficient discovery and coordinated proceedings while preserving individual claim values based on specific injuries—unlike class actions where all members receive identical compensation regardless of harm severity.
What Legal Experts Say
Legal analysts believe this will be an extremely successful mass tort for obtaining victim settlement compensation, given the strong scientific evidence linking Depo-Provera to meningioma.
A 5.6 times increased risk is not just a minor concern—it’s a flashing siren in mass tort cases, making it much easier for plaintiffs’ lawyers to prove claims and putting massive pressure on defendants.
Judge Rodgers is moving quickly to resolve litigation efficiently, with regular status conferences scheduled throughout 2025 as part of a strategy to expedite proceedings.
FAQs About Depo-Provera Lawsuits
What is the Depo-Provera lawsuit about?
Women are suing Pfizer alleging the birth control shot caused meningioma brain tumors. Over 1,346 federal cases claim Pfizer knew about tumor risks but failed to warn U.S. patients despite updating labels in Europe and Canada.
Can I file a claim if I used Depo-Provera years ago?
Yes. Statutes of limitations vary by state, but many allow claims years after diagnosis. Some states have “discovery rules” where the clock starts when you learned about the drug-tumor connection, not when you stopped injections.
How much compensation can I receive?
Projected settlements range from $150,000 to $800,000+ depending on injury severity. Cases requiring surgery, resulting in permanent neurological damage, or involving multiple tumors typically receive higher compensation.
What if I can’t find my medical records?
The court now requires third parties including pharmacies, clinics, hospitals, and insurance companies to provide injection records even when traditional medical records are unavailable. Your attorney can issue subpoenas for this documentation.
Do I need a lawyer to file?
Most Depo-Provera attorneys work on contingency (no upfront costs, payment only if you win). Given the complex medical evidence, MDL procedures, and preemption battles, legal representation significantly improves claim success.
What is the filing deadline?
Deadlines vary by state. Some states have statutes of limitations extending from diagnosis date or discovery of the drug connection. Consult an attorney immediately to preserve your rights—waiting could bar your claim.
How long will the lawsuit take?
Bellwether trials are expected late 2026 or early 2027. Settlement negotiations typically accelerate after early trial results. The preemption ruling expected soon will significantly impact timeline—favorable rulings expedite proceedings while unfavorable decisions could delay or dismiss cases.
Next Steps for Filing a Claim
1. Document Your Case: Gather all medical records, prescription history, diagnosis documentation, and treatment records showing Depo-Provera use and meningioma development.
2. Consult a Depo-Provera Attorney: Most firms offer free case evaluations and work on contingency, meaning no upfront costs. Attorneys experienced in pharmaceutical litigation understand MDL procedures and proof requirements.
3. Complete Questionnaires: Your attorney will help you complete the required Plaintiff Proof of Use/Injury Questionnaire within court deadlines, ensuring all necessary documentation is submitted properly.
4. Preserve Evidence: Keep all medical records, bills, employment documentation showing lost wages, and any correspondence with healthcare providers about your meningioma diagnosis and treatment.
5. Act Quickly: With nearly 10,000 potential unfiled claims and statutes of limitations varying by state, prompt action protects your right to compensation.
Legal Disclaimer: This article provides factual information about the Depo-Provera lawsuit based on publicly available court documents and news reports. It is for educational purposes only and does not constitute legal advice. Case details are based on allegations and court filings, which may not represent final determinations. Filing deadlines and eligibility requirements may vary. For legal advice regarding your specific situation or claim, please consult with a qualified attorney.
For more legal news and updates, visit All About Lawyer.
About the Author

Sarah Klein, JD, is a licensed attorney and legal content strategist with over 12 years of experience across civil, criminal, family, and regulatory law. At All About Lawyer, she covers a wide range of legal topics — from high-profile lawsuits and courtroom stories to state traffic laws and everyday legal questions — all with a focus on accuracy, clarity, and public understanding.
Her writing blends real legal insight with plain-English explanations, helping readers stay informed and legally aware.
Read more about Sarah
