Are Schedule 3 Drugs Legal? What You Need to Know and How Are They Regulated?
Schedule 3 drugs have moderate to low potential for physical and psychological dependence and are legal for medical use with a prescription in the United States. Federal law classifies substances like ketamine, testosterone, anabolic steroids, and products containing less than 90 milligrams of codeine as Schedule 3. Without a valid prescription, possession carries penalties up to one year imprisonment and fines up to $1,000 for first offenses.
What Is Schedule 3 Drug Classification?
The DEA defines Schedule 3 drugs as substances with accepted medical use but low to moderate addiction risk that is less than higher-scheduled drugs. Three formal criteria determine Schedule 3 placement:
- Abuse potential less than Schedule 1 and 2 drugs
- Currently accepted medical use in the United States
- Moderate to low risk of physical or psychological dependence
The DEA must make these findings through formal administrative proceedings before classifying any substance as Schedule 3.
Which Drugs Are Classified as Schedule 3?
Schedule 3 narcotics include products with less than 90 milligrams of codeine per dose and buprenorphine products, while non-narcotics include ketamine, anabolic steroids like testosterone and oxandrolone, and stimulants like benzphetamine and phendimetrazine.
Schedule 3 Narcotics:
- Codeine combinations (Tylenol with Codeine)
- Buprenorphine (Suboxone, Brixadi)
- Products with limited opioid content
Schedule 3 Non-Narcotics:
- Ketamine
- Anabolic steroids (testosterone, oxandrolone)
- Certain barbiturates
- Stimulants (benzphetamine, phendimetrazine)

Legal Status of Schedule 3 Drugs
Schedule 3 drugs are legal only with a valid prescription from a DEA-registered practitioner. Practitioners must have DEA registration numbers and prescriptions can be refilled up to 5 times within 6 months.
Possession without prescription authorization violates federal law under 21 USC 844. Distribution without proper licensing violates 21 USC 841 and carries significantly harsher penalties than simple possession.
Federal Penalties for Possession
First-time possession offenders face maximum sentences of 1 year imprisonment and fines up to $1,000, second offenses require minimum 15 days with maximum 2 years and fines up to $2,500, while third offenses mandate minimum 90 days with maximum 3 years and fines starting at $5,000.
The Anti-Drug Abuse Act of 1988 also imposes civil penalties separate from criminal prosecution. Individuals convicted may lose public housing assistance and student loan eligibility.
Federal Penalties for Distribution
First-offense distribution of Schedule 3 drugs carries penalties of not more than 5 years imprisonment and fines not exceeding $25,000 for individuals or $1 million for organizations.
Trafficking Schedule 3 substances brings up to 10 years imprisonment and $500,000 fines. When death or serious injury results from distributed drugs, maximum sentences increase to 15 years.
Legitimate Medical Uses and Prescribing
Licensed medical practitioners with DEA registration can prescribe Schedule 3 drugs for accepted medical purposes:
Buprenorphine: Opioid addiction treatment and chronic pain management
Testosterone and Anabolic Steroids: Hormone replacement therapy and specific medical conditions
Ketamine: Anesthesia and treatment-resistant depression
Codeine Combinations: Pain relief and cough suppression
Schedule 3 prescriptions may be refilled up to 5 times within a 6-month period from the prescription date. Prescriptions can be written, called in to pharmacies, or transmitted electronically through approved systems.
How State Laws Differ from Federal Law
Most states adopted controlled substance acts mirroring federal schedules, but penalties and enforcement vary significantly by jurisdiction.
California: Follows federal Schedule 3 classification under Health and Safety Code § 11056, referring to them as C-III drugs.
Pennsylvania: Simple possession charges as misdemeanor while possession with intent to deliver becomes felony with harsher fines and imprisonment.
Texas: Possession penalties range from up to 1 year or 3 years depending on offense number, with fines from $1,000 to $3,000.
Alabama and Ohio: State penalties for Schedule 3 distribution include up to 7 years imprisonment and fines up to $5,000.
States maintain independent authority to impose additional restrictions beyond federal requirements. Some states mandate electronic prescribing for controlled substances, while federal law makes it voluntary.
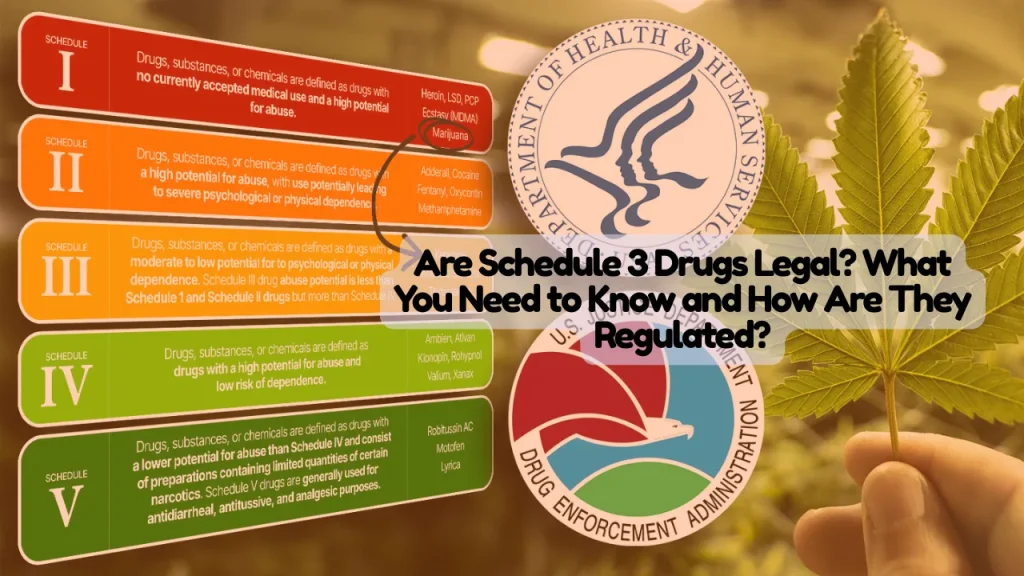
DEA Scheduling Framework Explained
The Controlled Substances Act of 1970 established five schedules ranking drugs by abuse potential and medical utility. Schedule 1 substances have highest abuse potential with no medical use, Schedule 2 have high abuse potential but accepted medical uses, while Schedule 3 through 5 progressively decrease in restriction.
Two federal agencies control scheduling decisions: the DEA and FDA jointly determine which substances are added or removed from schedules, though Congress can directly schedule substances through legislation.
The DEA considers multiple factors: pharmacological effects, scientific evidence, public health risks, abuse trends, and whether minor chemical modifications could increase danger.
Schedule 3 vs. Other Drug Schedules
Schedule 1: No accepted medical use, highest abuse potential (heroin, LSD, marijuana federally)
Schedule 2: High abuse potential, severe dependence risk, accepted medical use with strict controls (fentanyl, cocaine, methamphetamine, Adderall)
Schedule 3: Moderate-to-low abuse potential, accepted medical use, refills permitted (ketamine, testosterone, codeine combinations)
Schedule 4: Lower abuse potential than Schedule 3 (Xanax, Valium, Ambien)
Schedule 5: Lowest abuse potential with limited narcotic quantities (cough preparations with minimal codeine)
Schedule 2 prescriptions cannot be refilled and require written or electronic submission. Schedule 3 and 4 drugs allow refills, making them more accessible for ongoing treatment.
Related article: Is Jaywalking Legal in California? What the Freedom to Walk Act Allows
Recent Changes in Drug Scheduling
The most significant pending change involves marijuana reclassification. President Trump is expected to sign an executive order reclassifying marijuana from Schedule 1 to Schedule 3, which would ease federal restrictions though not fully legalize the drug.
The Biden administration initiated marijuana rescheduling in October 2022. In August 2023, HHS recommended marijuana transfer from Schedule 1 to Schedule 3 based on scientific and medical evaluation, and DEA proposed this rescheduling in May 2024.
A hearing scheduled for January 21, 2025 was postponed pending appeal resolution. The DEA received over 42,000 public comments on the proposal. The DEA selected more than 20 hearing participants in October 2024, including scientists, lawyers, doctors, and both supporters and opponents of marijuana legalization.
If finalized, marijuana rescheduling would allow medical research and potentially reduce criminal penalties, but state-authorized marijuana dispensaries would remain federally unauthorized since Schedule 3 drugs require FDA approval and DEA-registered pharmacy dispensing.
How Schedule 3 Drugs Are Enforced
Federal enforcement focuses primarily on trafficking, diversion from legitimate medical channels, and large-scale illegal distribution. State and local law enforcement handle most possession cases.
The DEA maintains registration systems tracking all authorized handlers of controlled substances. Manufacturers, distributors, researchers, pharmacies, and prescribers must obtain DEA registration and maintain detailed records of all transactions.
Prescription Drug Monitoring Programs (PDMPs) operate in 49 states plus DC and Guam, electronically tracking controlled substance prescriptions to identify potential abuse or diversion. Pharmacists and prescribers can access PDMPs to verify patient prescription histories.
Prescribing Requirements for Medical Professionals
All practitioners prescribing Schedule 3 drugs must hold valid DEA registration numbers. Registration requirements include:
- Professional license or certificate in good standing
- State authorization to prescribe controlled substances
- Completion of training for certain medications
- Maintenance of patient evaluation and prescribing records
- Implementation of controls against diversion
DEA registration numbers contain 2 letters followed by 7 numbers. The first letter indicates registration type (A or B for most practitioners, M for mid-level practitioners like nurse practitioners).
Practitioners can issue Schedule 3 prescriptions in written form, via phone to pharmacies, or through electronic prescribing systems. Unlike Schedule 2 drugs requiring written prescriptions, Schedule 3 allows verbal orders to be called in.
Legal Rights and Protections
Individuals charged with Schedule 3 possession or distribution offenses have constitutional rights to legal representation, due process, and trial.
Legitimate prescription holders cannot be prosecuted for possession of prescribed medications taken according to medical directions. Patients should maintain prescription records and ensure medications remain in original labeled containers.
Medical necessity defenses may apply when prescribed Schedule 3 drugs treat documented conditions under proper medical supervision. Courts evaluate whether prescriptions serve legitimate medical purposes rather than drug-seeking behavior.
Pharmacists have “corresponding responsibility” to verify prescription legitimacy before dispensing. If prescriptions raise red flags regarding quantity, frequency, or patient behavior, pharmacists can refuse to fill them.
Marijuana Rescheduling Impact
Moving marijuana to Schedule 3 would place it in the same class as ketamine, Tylenol with codeine, and testosterone, making it available by prescription subject to FDA approval and DEA-registered pharmacy dispensing.
However, rescheduling does not legalize state marijuana programs. Dispensaries operating under state law would remain federally unauthorized since Schedule 3 drugs require FDA approval that marijuana currently lacks.
Research restrictions would ease substantially. Scientists could more easily study marijuana’s medical applications without navigating Schedule 1 research barriers.
Tax implications could change significantly. Current IRS Code Section 280E prevents marijuana businesses from deducting ordinary business expenses. Schedule 3 status might allow standard tax treatment, though legal interpretation remains uncertain.
Criminal penalties would decrease but not disappear. Unauthorized possession and distribution would still violate federal law, though courts might impose lighter sentences than for Schedule 1 substances.
Frequently Asked Questions
Can I legally possess Schedule 3 drugs without a prescription?
No. Federal law prohibits possession of Schedule 3 drugs without valid prescriptions from DEA-registered practitioners. First-offense possession carries up to 1 year imprisonment and $1,000 fines.
How many times can Schedule 3 prescriptions be refilled?
Schedule 3 prescriptions can be refilled up to 5 times within 6 months from the prescription date. After that period or 5 refills (whichever comes first), a new prescription is required.
Do state penalties differ from federal penalties for Schedule 3 drugs?
Yes. States independently establish controlled substance penalties. Some impose harsher sentences than federal minimums, while others may offer diversion programs or reduced charges for first-time offenders.
What happens if marijuana is reclassified to Schedule 3?
Marijuana would gain recognition for accepted medical use, allowing easier research and potentially reducing federal criminal penalties. However, state dispensaries would remain federally unauthorized without FDA approval.
Can doctors prescribe Schedule 3 drugs via telemedicine?
Yes, with restrictions. Practitioners can prescribe Schedule 3 drugs via telemedicine if they conduct proper patient evaluations using real-time audio-visual communication and maintain legitimate doctor-patient relationships.
What is the difference between Schedule 3 possession and distribution charges?
Possession charges apply when individuals hold drugs for personal use, carrying maximum 1-year sentences for first offenses. Distribution charges involve selling or transferring drugs to others, carrying up to 5 years imprisonment and significantly higher fines.
Are anabolic steroids illegal without a prescription?
Yes. Anabolic steroids are Schedule 3 controlled substances requiring prescriptions for legitimate medical purposes. Illegal possession, distribution, or use for athletic performance enhancement violates federal law.
Sources:
- DEA Drug Scheduling
- 21 USC 812: Schedules of Controlled Substances
- 21 CFR 1308.13: Schedule III Regulations
- DEA Controlled Substance Schedules
Last Updated: December 18, 2025
About the Author

Sarah Klein, JD, is a former criminal defense attorney with hands-on experience in cases involving DUIs, petty theft, assault, and false accusations. Through All About Lawyer, she now helps readers understand their legal rights, the criminal justice process, and how to protect themselves when facing charges.
Read more about Sarah
