DePuy Attune Knee Lawsuit Exposed, 400,000+ Implants at Risk—Active Claims, Zero MDL & What 2025 Really Means for Compensation
DePuy Attune knee lawsuits remain active in federal and state courts across the United States with no multidistrict litigation established, leaving individual plaintiffs to pursue claims against Johnson & Johnson through separate actions. More than 400,000 patients received Attune knee implants since FDA approval in 2010, but no large group settlements have been reached as of 2025. The first lawsuit was filed in Alabama in September 2017, and cases continue emerging as patients experience implant failures requiring painful revision surgeries.
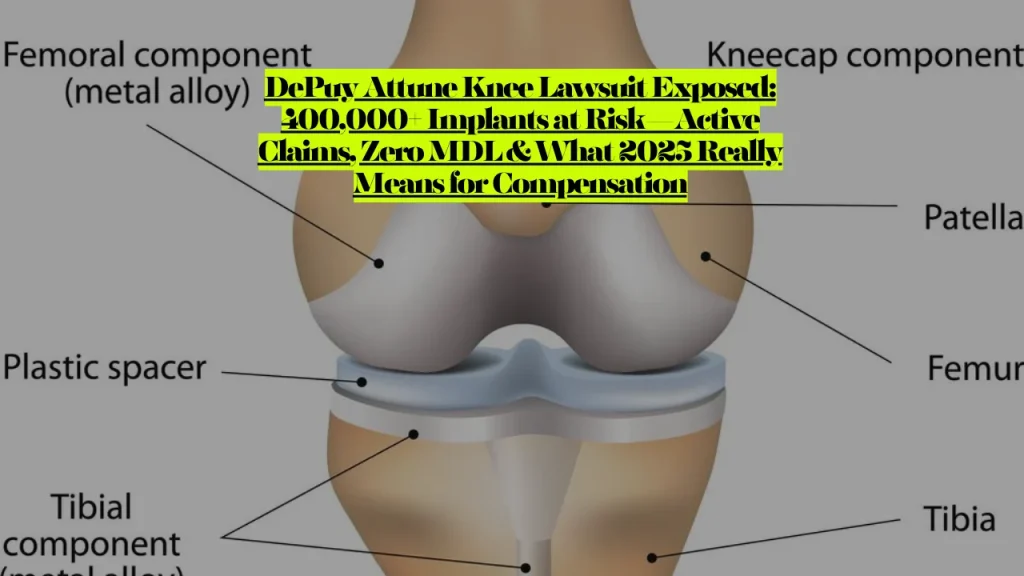
The central defect involves tibial component debonding—surgical cement failing to adhere to the implant’s unusually smooth metal surface, causing the device to loosen from the tibia bone within two years of implantation. The FDA’s MAUDE database documented over 1,400 adverse event reports, with more than 650 requiring revision surgery.
Which DePuy Attune Lawsuits Are Currently Active?
Individual product liability claims proceed in state and federal courts nationwide. A 2023 lawsuit filed in U.S. District Court for the Western District of North Carolina alleges DePuy downplayed risks and never recalled the defective tibial baseplate despite significantly higher failure rates than competing devices.
Active litigation targets:
- Fixed-bearing tibial component failures: Posterior-stabilized and cruciate-retaining models experience cement loosening at higher rates, typically within two years post-surgery
- Design defect claims: Testing reveals the Attune surface is 75% smoother than comparable knee prosthetics, preventing adequate cement adhesion
- Failure to warn allegations: Lawsuits claim DePuy never informed doctors or patients about debonding risks despite internal knowledge of the defect
In 2018, David Love filed suit in Jackson County, Missouri, after experiencing persistent pain and instability for two years before revision surgery revealed a “failed tibial component” that had become “grossly loose.”
What Is the Current Settlement Status?
As of 2024, there have been no large group settlements involving Attune knee failures. Unlike the $2.5 billion DePuy paid to settle 8,000 ASR hip implant cases in 2013, Attune litigation has not reached settlement stage.
Why no MDL exists:
No federal panel has consolidated Attune cases into multidistrict litigation. Without MDL coordination, cases proceed independently, delaying bellwether trials that typically drive settlement negotiations. Plaintiffs pursue compensation through individual state and federal lawsuits without unified discovery or coordinated legal strategy.
Historic knee replacement settlement context:
Sulzer Medica paid $1 billion to settle 4,000 hip and knee implant cases—the largest knee replacement settlement on record. Zimmer NexGen knee litigation involved more than 1,700 federal lawsuits before reaching confidential settlement in February 2018.
Who Qualifies for DePuy Attune Compensation Claims?
Primary eligibility criteria:
- Received DePuy Attune Total Knee Arthroplasty system (TKA) with fixed-bearing tibial component
- Experienced tibial baseplate loosening, debonding, or implant instability
- Required or were advised to undergo revision surgery to remove failed implant
- Documented medical records showing Attune-specific complications
Qualifying complications include:
- Persistent pain beyond normal recovery period, instability causing loss of balance or range of motion
- Swelling and inflammation at surgical site, infection or tissue damage from loose implant
- Early failure requiring revision within 2-12 years (normal lifespan: 12-20 years)
While no official recall exists, DePuy modified the design and now offers revision-specific knee systems—suggesting the company acknowledged performance issues.
What Are Realistic Compensation Amounts?
Prior knee replacement settlements have ranged between $50,000 and $500,000, with some substantially larger payouts involving extraordinary injuries. Settlement value depends on multiple factors.
Damages typically sought:
Economic losses:
- Past and future medical expenses (revision surgery, post-operative care, physical therapy)
- Lost wages and diminished earning capacity
- Out-of-pocket costs for home care assistance
Non-economic damages:
- Pain and suffering from failed implant
- Loss of quality of life and mobility limitations
- Emotional distress from repeat surgeries
Punitive damages:
- If evidence proves DePuy knew about the design defect but failed to warn patients or issue recalls, courts may award punitive damages to punish reckless conduct
In DePuy’s 2013 hip implant settlement, persons implanted with faulty devices were awarded an average of over $300,000 each for pain, medical bills, and rehabilitation.
What Recent Court Developments Affect Claims?
2023-2024 litigation activity:
April 2023: Federal lawsuit filed in Western District of North Carolina alleging DePuy knew about significantly higher failure rates but never recalled the tibial baseplate or informed medical professionals.
2017: Journal of Knee Surgery published study documenting unusually high premature failure rates, with surgeons attributing failures to debonding at the tibial implant-cement interface.
DePuy’s documented acknowledgment:
DePuy Synthes admitted in response to the Journal of Knee Surgery study that the Attune tibial base plate’s roughness factor may be three times higher than the comparable Sigma tibial base plate—effectively acknowledging the design flaw driving cement adhesion failures.
What Documentation Strengthens Your Claim?
Critical evidence needed:
Medical records proving:
- Surgery date and confirmation of Attune implant model
- X-rays showing tibial loosening or implant failure
- Revision surgery reports documenting failed component
- Post-operative complications and treatment history
Additional supporting documentation:
- Implant identification card provided at surgery
- FDA adverse event reports filed by your surgeon
- Expert testimony from orthopedic surgeons about debonding defects
- Financial records of medical expenses and lost income
A 2017 study found 15 failures at just three hospitals, with only 2 visible on X-ray—meaning 13 failures eluded detection and could only be identified through patient symptoms and complaints. Document all persistent pain, instability, and mobility issues even if imaging appears normal.
What Are the Statute of Limitations and Filing Deadlines?
Timing considerations:
Most states impose 1-3 year statutes of limitation for product liability claims, typically starting when you discover (or should have discovered) the defect caused your injury—not the original surgery date.
Discovery rule application:
Many patients don’t realize their Attune knee failed due to a design defect rather than normal surgical complications. Courts often apply the “discovery rule,” starting the statute of limitations when you learned the implant was defective, not when you first experienced symptoms.
Why prompt action matters:
Several law firms have stopped accepting new DePuy Attune cases after years of litigation, though active firms continue investigating claims for patients who experienced tibial component failures.
How Do You File a DePuy Attune Lawsuit?
Claim process steps:
1. Confirm your implant model Contact your orthopedic surgeon or hospital to verify you received a DePuy Attune knee system with fixed-bearing tibial component.
2. Gather comprehensive medical records Obtain all documentation related to your knee replacement, complications, revision surgery, and ongoing treatment.
3. Consult product liability attorneys Seek firms with proven track record in medical device litigation. Look for firms that have received jury verdicts of $1 million or more and achieved settlements exceeding $4 billion in similar cases.
4. Case evaluation and filing Experienced attorneys review your medical history, determine eligibility, and file suit in appropriate state or federal court.
Contingency fee arrangements:
Most product liability attorneys handle Attune cases on contingency—you pay no fees unless you win. Legal costs are typically deducted from settlement or verdict proceeds.
What Common Misconceptions Should You Avoid?
Myth: Only revision surgery patients qualify
Reality: If your doctor advised revision surgery but you haven’t undergone it yet, you may still have a valid claim. Document the recommendation and continue monitoring your condition.
Myth: FDA approval means DePuy isn’t liable
Reality: The Attune received FDA approval through the 510(k) fast-track program based on “substantial equivalence” to existing devices—requiring no clinical trials or independent safety evidence. One comparable device, Zimmer’s NexGen CR Knee System, was later subject to a Class 2 recall in January 2016.
Myth: All knee pain qualifies for compensation
Reality: Claims require proof that Attune-specific design defects (tibial debonding) caused your injury, not general surgical complications or pre-existing conditions.
Myth: Settlements are imminent
Reality: Large group settlements typically occur only after several cases are tried before juries, allowing manufacturers to assess their financial risk. Without an established MDL or bellwether trial results, Attune settlements remain uncertain.
Frequently Asked Questions
What is the latest DePuy Attune knee lawsuit status in 2025?
Individual lawsuits remain active in state and federal courts with no multidistrict litigation established and no large group settlements reached. Plaintiffs continue filing claims for tibial component failures requiring revision surgery.
How much compensation can I receive from a DePuy Attune claim?
Prior knee replacement settlements ranged from $50,000 to $500,000, with some substantially larger payouts for extraordinary injuries. Your specific compensation depends on medical expenses, lost wages, pain and suffering, and whether punitive damages apply.
Which DePuy Attune models are affected by the debonding defect?
The DePuy Synthes Attune posterior-stabilized fixed-bearing knee implant and cruciate-retaining fixed-bearing knee prosthetic are particularly prone to cement loosening issues.
What is the eligibility criteria for filing an Attune lawsuit?
You must have received an Attune TKA system with fixed-bearing tibial component, experienced loosening or debonding complications, and required (or been advised to undergo) revision surgery. Medical records must document Attune-specific failures.
How long do I have to file a DePuy Attune lawsuit?
Most states impose 1-3 year statutes of limitation starting when you discovered the implant defect caused your injury. Consult an attorney promptly to preserve your rights, as deadlines vary by state.
Why hasn’t DePuy recalled the Attune knee implant?
While no official recall exists, DePuy modified the design and offers revision-specific systems. Lawsuits allege the company never recalled the tibial baseplate despite internal knowledge of higher failure rates.
What makes the Attune different from other knee implants?
Testing shows the Attune surface is 75% smoother than comparable knee prosthetics, preventing surgical cement from adequately bonding to the tibial component. This design flaw leads to premature loosening typically within two years.
Take Action If You Have an Attune Implant
If you received a DePuy Attune knee replacement and experienced early failure, persistent pain, instability, or required revision surgery, consult with experienced product liability attorneys immediately. Document all symptoms and complications, preserve your medical records, and act before statute of limitations deadlines expire.
This information is for educational purposes only and does not constitute legal advice. DePuy Attune claim eligibility and compensation amounts vary by individual circumstances and applicable litigation terms. Consult official claims administrator resources or a qualified attorney for specific guidance regarding your eligibility and potential compensation.
Related Articles:
- Questions to Ask a Probate Lawyer Before Hiring
- How Much Does a Product Liability Lawyer Cost
- Should I Get a Lawyer for Identity Theft
Sources:
- Journal of Knee Surgery (June 2017) study on Attune knee failures
- FDA MAUDE database adverse event reports
- U.S. District Court filings (Western District of North Carolina, Mississippi, Alabama)
- DePuy Synthes official responses to failure rate studies
- Product liability case settlements and verdict records
About the Author

Sarah Klein, JD, is a licensed attorney and legal content strategist with over 12 years of experience across civil, criminal, family, and regulatory law. At All About Lawyer, she covers a wide range of legal topics — from high-profile lawsuits and courtroom stories to state traffic laws and everyday legal questions — all with a focus on accuracy, clarity, and public understanding.
Her writing blends real legal insight with plain-English explanations, helping readers stay informed and legally aware.
Read more about Sarah
